Research Overview
HiTS research accelerates the invention of the life-saving medicines and precision diagnostics needed to improve patient care.
Historically, the different phases of therapeutic discovery, development, evaluation, and delivery have been siloed. We take a fundamentally new approach that fuses laboratory investigation, computer science, and clinical research. We partner with clinicians to make sure that research projects address medically relevant questions. Insights from our laboratory studies go on to inform drug development, clinical trial design, and regulatory evaluation through partnerships with pharmaceutical companies, hospitals, and the FDA.
Our approach
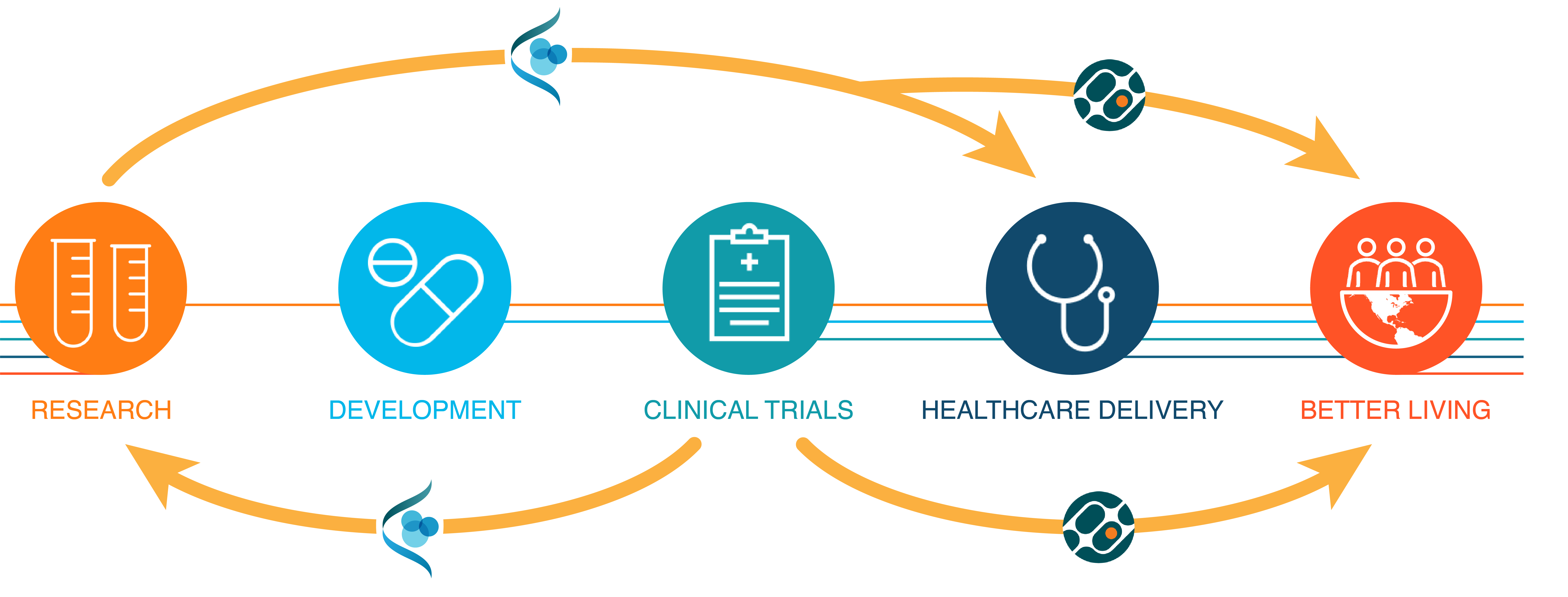
Areas of Research
The Laboratory of Systems Pharmacology (LSP)
The Laboratory of Systems Pharmacology uses novel approaches to study the changes that cause disease and how therapeutic drugs function at the molecular level.
Research areas include:
- Diagnostics and biomarkers
- Cancer immunology
- Therapeutic resistance & sensitivity
- Inflammation in disease
- Clinical trials
- Understanding and treating infection
- Immunotherapy response
- ... and more!
The Harvard-MIT Center for Regulatory Science
The Center for Regulatory Science applies an interdisciplinary approach to examine the processes that classify drugs and devices as safe and effective.
Research areas include:
- Surveillance of post-market medical device safety
- Assessment, uptake, and applications of digital health technologies
- Pediatric medical device development
- Cell and gene therapy
- Applying real-world evidence to regulatory decision making
- ... and more!
